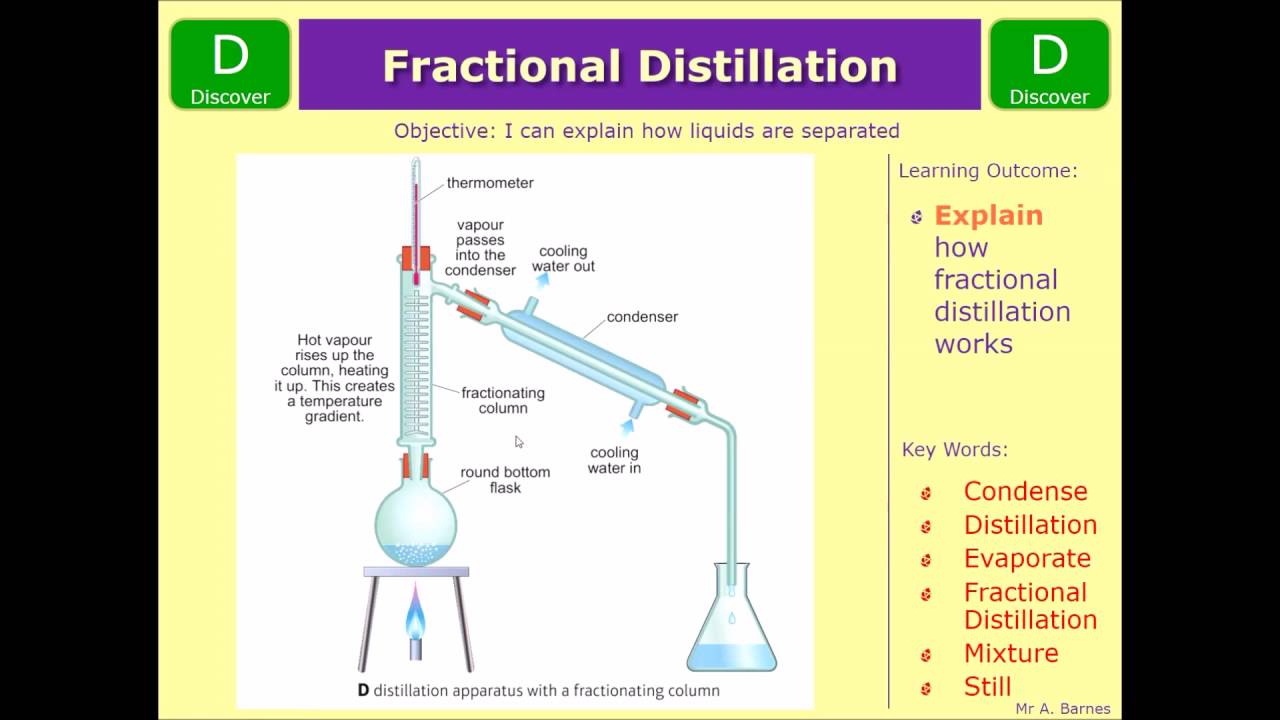
How Does Fractional Distillation Work Simple . Fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and. We talked about how you would set up a simple distillation and how that's great for separating. A simple distillation is incapable of significant purification if the boiling points of the components are too close. The distillate of a simple distillation is always enriched in the lower boiling. Fractional distillation is a type of distillation which involves the separation of miscible liquids. Learn how to separate and analyse chemicals using filtration; This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram.
from www.youtube.com
A simple distillation is incapable of significant purification if the boiling points of the components are too close. The distillate of a simple distillation is always enriched in the lower boiling. Fractional distillation is a type of distillation which involves the separation of miscible liquids. Fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and. Learn how to separate and analyse chemicals using filtration; We talked about how you would set up a simple distillation and how that's great for separating. This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram.
GCSE Chemistry 19 Simple and Fractional Distillation YouTube
How Does Fractional Distillation Work Simple A simple distillation is incapable of significant purification if the boiling points of the components are too close. A simple distillation is incapable of significant purification if the boiling points of the components are too close. This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. The distillate of a simple distillation is always enriched in the lower boiling. Fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and. Learn how to separate and analyse chemicals using filtration; Fractional distillation is a type of distillation which involves the separation of miscible liquids. We talked about how you would set up a simple distillation and how that's great for separating.
From www.pinterest.com
distillation tower, illustration Google Search Fractional How Does Fractional Distillation Work Simple The distillate of a simple distillation is always enriched in the lower boiling. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Fractional distillation is a type of distillation which involves the separation of miscible liquids. We talked about how you would set up a simple distillation and how that's great. How Does Fractional Distillation Work Simple.
From www.pinterest.ca
Best 25+ Fractional distillation ideas on Pinterest Crude oil How Does Fractional Distillation Work Simple We talked about how you would set up a simple distillation and how that's great for separating. Learn how to separate and analyse chemicals using filtration; Fractional distillation is a type of distillation which involves the separation of miscible liquids. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Fractional distillation. How Does Fractional Distillation Work Simple.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram How Does Fractional Distillation Work Simple The distillate of a simple distillation is always enriched in the lower boiling. This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. Fractional distillation is a type of distillation which involves the separation of miscible liquids. Learn how to separate and analyse chemicals using filtration;. How Does Fractional Distillation Work Simple.
From chemicalengineeringworld.com
Fractional Distillation Process Chemical Engineering World How Does Fractional Distillation Work Simple Learn how to separate and analyse chemicals using filtration; This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. The distillate of a simple distillation is always enriched in the lower boiling. We talked about how you would set up a simple distillation and how that's. How Does Fractional Distillation Work Simple.
From www.slideshare.net
Fractional distillation How Does Fractional Distillation Work Simple This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Learn how to separate and analyse chemicals using filtration; Fractional distillation is used in oil refineries (figure 5.41). How Does Fractional Distillation Work Simple.
From www.youtube.com
Introduction to Fractional distillation Distillation procedure Home How Does Fractional Distillation Work Simple The distillate of a simple distillation is always enriched in the lower boiling. A simple distillation is incapable of significant purification if the boiling points of the components are too close. This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. Learn how to separate and. How Does Fractional Distillation Work Simple.
From www.vectorstock.com
Diagram showing fractional distillation crude oil Vector Image How Does Fractional Distillation Work Simple Learn how to separate and analyse chemicals using filtration; The distillate of a simple distillation is always enriched in the lower boiling. We talked about how you would set up a simple distillation and how that's great for separating. Fractional distillation is a type of distillation which involves the separation of miscible liquids. This page explains how the fractional distillation. How Does Fractional Distillation Work Simple.
From www.slideserve.com
PPT Fractional Distillation PowerPoint Presentation ID6021367 How Does Fractional Distillation Work Simple A simple distillation is incapable of significant purification if the boiling points of the components are too close. Fractional distillation is a type of distillation which involves the separation of miscible liquids. We talked about how you would set up a simple distillation and how that's great for separating. This page explains how the fractional distillation (both in the lab. How Does Fractional Distillation Work Simple.
From www.vectorstock.com
Diagram showing fractional distillation crude oil Vector Image How Does Fractional Distillation Work Simple Learn how to separate and analyse chemicals using filtration; Fractional distillation is a type of distillation which involves the separation of miscible liquids. The distillate of a simple distillation is always enriched in the lower boiling. We talked about how you would set up a simple distillation and how that's great for separating. This page explains how the fractional distillation. How Does Fractional Distillation Work Simple.
From www.chemicals.co.uk
What is Fractional Distillation? The Chemistry Blog How Does Fractional Distillation Work Simple Learn how to separate and analyse chemicals using filtration; We talked about how you would set up a simple distillation and how that's great for separating. This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. Fractional distillation is a type of distillation which involves the. How Does Fractional Distillation Work Simple.
From chembam.com
Fractional Distillation How Does Fractional Distillation Work Simple Learn how to separate and analyse chemicals using filtration; Fractional distillation is a type of distillation which involves the separation of miscible liquids. We talked about how you would set up a simple distillation and how that's great for separating. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Fractional distillation. How Does Fractional Distillation Work Simple.
From www.vernier.com
Fractional Distillation > Experiment 8 from Chemistry with Vernier How Does Fractional Distillation Work Simple Fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and. We talked about how you would set up a simple distillation and how that's great for separating. Fractional distillation is a type of distillation which involves the separation of miscible liquids. The distillate of a simple distillation is. How Does Fractional Distillation Work Simple.
From brainly.in
Draw a labelled diagram showing the fractional distillation of How Does Fractional Distillation Work Simple A simple distillation is incapable of significant purification if the boiling points of the components are too close. Fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and. Learn how to separate and analyse chemicals using filtration; We talked about how you would set up a simple distillation. How Does Fractional Distillation Work Simple.
From www.elevise.co.uk
C1 I) Simple & Fractional Distillation AQA Combined Science Trilogy How Does Fractional Distillation Work Simple The distillate of a simple distillation is always enriched in the lower boiling. We talked about how you would set up a simple distillation and how that's great for separating. Fractional distillation is a type of distillation which involves the separation of miscible liquids. Fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions. How Does Fractional Distillation Work Simple.
From www.youtube.com
Fractional Distillation using column 11th Std Chemistry Science How Does Fractional Distillation Work Simple This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. Fractional distillation is used in oil refineries (figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and. The distillate of a simple distillation is always enriched in the lower boiling. A. How Does Fractional Distillation Work Simple.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog How Does Fractional Distillation Work Simple A simple distillation is incapable of significant purification if the boiling points of the components are too close. We talked about how you would set up a simple distillation and how that's great for separating. Learn how to separate and analyse chemicals using filtration; Fractional distillation is a type of distillation which involves the separation of miscible liquids. This page. How Does Fractional Distillation Work Simple.
From www.youtube.com
GCSE Chemistry 19 Simple and Fractional Distillation YouTube How Does Fractional Distillation Work Simple Fractional distillation is a type of distillation which involves the separation of miscible liquids. The distillate of a simple distillation is always enriched in the lower boiling. We talked about how you would set up a simple distillation and how that's great for separating. This page explains how the fractional distillation (both in the lab and industrially) of an ideal. How Does Fractional Distillation Work Simple.
From psiberg.com
Simple vs. Fractional Distillation A Comparison How Does Fractional Distillation Work Simple We talked about how you would set up a simple distillation and how that's great for separating. This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Learn. How Does Fractional Distillation Work Simple.